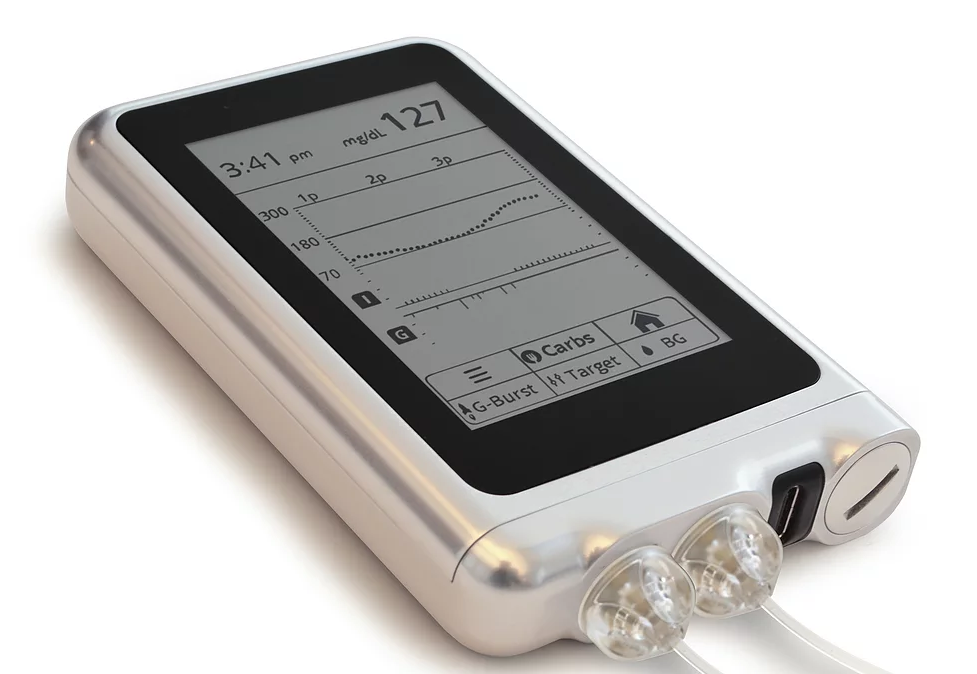
Bionic Pancrease
Services Provided
Program / Project Management
Risk Management
Regulatory / Quality
Systems Engineering
Verification & Validation
Client Background:
For multiple reasons, this pharmaceutical client chose to transition away from their current design service partner and move the project’s management to our team.
Client’s Request(s):
The client requested we progress the project toward a clinical trial, leveraging the existing design and any previously-manufactured components as much as possible.
They also asked us to assist with the management of any outsourced manufacturing of the device’s circuit cards and overall box build.
Project Actions:
This project delivered the services listed above, the development of several test fixtures, and provided both resources and expertise in Systems Design, Electrical, Mechanical, Software, and Manufacturing engineering.
Other highlights:
Led a team of 10 engineers and technicians to revise and finalize the design
Added new Bluetooth LE programming to the device
Sourced new electronics supplier
Produced 150 flex circuit assemblies in preparation for clinical trial
Programmed, diagnosed, tested, and assembled over 130 final assemblies
Delivered 35+ test fixtures for use in a drug manufacturer’s clinical trial
Outcome:
Clinical trials passed with the designed test fixtures in both drug clinical trials as well as device clinical trials.
As a result of this collaboration and the successful completion of both clinical trials, the client won another 60 million in funding.